- HOME
- Products and Services
- Plant Engineering
- Pharmaceutical and Life Science
- Pharmaceutical Plants
Pharmaceutical Plants

A dedicated team manages each project from design through to construction and handover to meet customer needs with an even higher level of support. We provide an integrated service for manufacturing equipment and construction compliant with the latest PIC/S and GMP* systems.
- PIC/S: Pharmaceutical Inspection Co-operation Scheme that promotes globalization of GMP
GMP: Good Manufacturing Practice system for ensuring product safety and consistent quality through all processes, from receipt of raw materials to shipment of products
Examples
Bulk pharmaceutical plant

Scope of service
- Design, equipment procurement, construction, installation, trial operation, validation
Features
- Compliant with the latest PIC/S and GMP systems
- Multi-product multi-purpose pharmaceutical plant
- Assistance in creating user requirement specifications (URS)
- Computerized System Validation (CSV) construction support and production control system installation
Bulk and investigational pharmaceutical plant

Scope of service
- Design, equipment procurement, construction, installation, trial operation, validation
Features
- Compliant with United States Food and Drug Administration (FDA) approval
- Switchable type, multi-purpose piping system
- Cross-contamination prevention
- Excellent cleanability
- Highly expandable
Extract powder plant
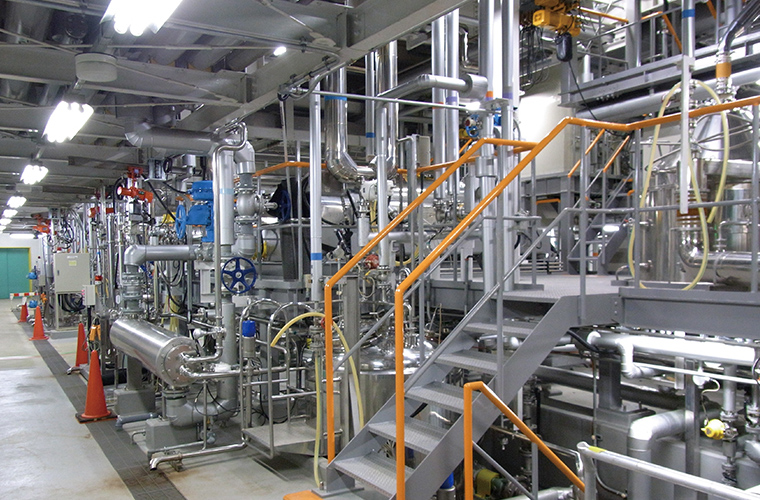
Scope of service
- Design, equipment procurement, construction, trial operation, validation
Features
- Equipment designed to suit characteristics of products
- Large spray dryer
- Equipment configuration for outstanding cleanability
- Faster type changeovers and reduced workload
Biopharmaceutical material refining plant

Scope of service
- Design, equipment procurement, construction, installation, trial operation, validation (including IQ and OQ)
Features
- Integrated plant, from mixing to polymerization, cleaning, filtering, and filling
- Plant layout designed for visitor inspections
- Temperature-controlled hazardous raw material warehouse and product warehouse attached
Biopharmaceutical product refining plant

Scope of service
- Design, equipment procurement, construction, installation, trial operation, validation (including IQ and OQ)
Features
- High-precision column chromatography refinement equipment
- Zoning and isolation
- Future potential-oriented plant layout (additional space provided within buildings and premises)
Pharmaceutical intermediate plant

Scope of service
- Design, equipment procurement, construction, installation, trial operation, validation
Features
- Business-oriented plant combining commercial, pilot, and R&D functions
- Equipment configuration, and selection of corrosion-resistant materials, for high-variety production
- Use of structures that allow for future building expansion